Background:
Approximately 40% of patients with diffuse large B-cell lymphoma (DLBCL) are >70 years old and have poor clinical outcomes attributable to treatment-related factors or to disease biology. Genotyping data in elderly DLBCL patients is limited because of lack of representation in clinical trials. R-mini-CHOP is better tolerated than R-CHOP in elderly DLBCL but with reduced efficacy. The addition of ibrutinib to R-CHOP has been shown to be effective in younger pts aged < 60 years but is associated with poor tolerability and outcomes in older patients. The ALLG Ibrutinib with R-mini-CHOP (IRiC) study, a prospective multicentre single-arm phase II study in elderly (≥ 75 years) DLBCL patients provided an opportunity to genotype this uncommon cohort.
We therefore aimed to map the genetic landscape of mutations in elderly DLBCL and compare them to a non-trial younger cohort.
Methods:
Mutations in genes commonly associated with DLBCL were assessed in 55 IRIC study patients with a median age of 81 years (75-91 years) and a gender ratio of 1.0 (27 M/28 F). A cohort of 51 non-trial patients withde novoDLBCL treated with anthracycline-based regimens was also genotyped. The median age of the control group was 65 years (29-91 years) with a gender ratio of 1.2:1 (28 M/23 F).
The cell of origin (COO) measured using gene expression profiling on 39/55 trial patients (non-GC=13, GC= 22, unclassified= 4), and Hans algorithm on 45/51 non-trial patients (non-GC=14, GC=31) was comparable (p=0.571).
Outcome data was available at a median follow-up of 18 and 40 months for the trial and non-trial cohorts respectively.
We extracted genomic DNA from and performed next generation sequencing on diagnostic formalin-fixed paraffin-embedded or fresh frozen tissue samples using a customized capture library (SureSelectXT Target Enrichment System, Aqilent Technologies) covering genes involved in lymphomagenesis. The purified libraries were sequenced on the Illumina NextSeq500 platform at AGRF (Australian Genome Research Facility, Australia).
Mutations in the following genes were compared across the two cohorts: ARID1A, BCL2, BTG1, BTG2, CARD11, CCND3, DTX1, EP300, ETS1, EZH2, FOXO1, GNA13, HIST1H1C, IKBKB, IRF8, KDM2B, KLHL6, MYC, MYD88, NOTCH1, NOTCH2, PIK3CD, PIM1, PRDM1, PTEN, PTPN21, SGK1, SPEN, STAT3, TET2, TNFAIP3, TNFRSF11A, TNFRSF14, TP53 and TRAF5.
Statistical analysis for nominal data was done using the chi-square test and for ordinal data using the Kruskal-Wallis test (p < 0.05= significant). Kaplan-Meier curves were calculated for patients with and without each mutation and the curves compared using a log-rank approach.
Results:
Patients were divided into 2 groups, IRiC trial cohort aged >75 years (n=55) + non-trial elderly patients >75 years (n=9) =elderly cohort(n=64) and non-trialnon-elderly cohortof patients < 75 years (n=42).
As expected, elderly patients were more likely to have high-risk disease with higher IPI of ≥ 3 (n=42, n=17, p=0.009). There was no significant difference in gender or COO.
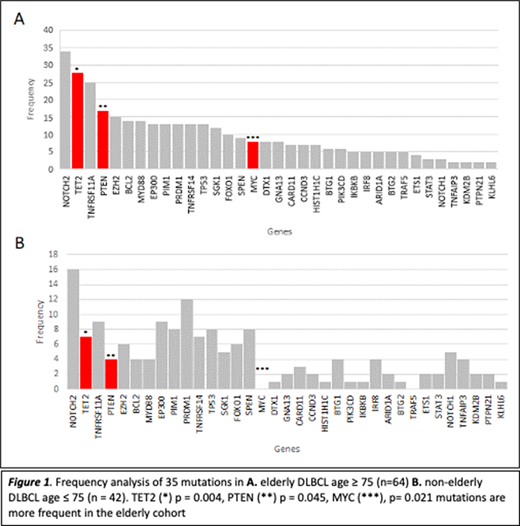
The frequency of mutations in the elderly (n=64) was compared to the non-elderly cohort (n=42). NOTCH2 was the most common mutation irrespective of age (34 [53%]; 16 [38%], p=0.129). Notably, we found that mutations in MYC (8 [12.5%]; 0, p= 0.021), PTEN (17 [26.5%]; 4 [9%], p=0.045) and TET2 (28 [43.7%]); 7 [16.6%], p=0.004) were more frequent in the elderly.
As expected, MYD88 and CD79B mutations were more frequently associated with non-GC subtype (p=0.001). No other associations with COO were identified.
No clear prognostic individual genes or gene clusters could be identified in the trial or the elderly cohort. Ibrutinib-responsive (MYD88 [L265P n=7] and CD79B [n=11]) and ibrutinib-resistant mutations (CARD11 [n=6] or PIM1 [n=12]) did not show clear associations with response rate, overall survival or progression-free survival.
Conclusions:
Our study found that the mutational profile of elderly DLBCL patients aged ≥ 75 years is enriched for targetable mutations in MYC, PTEN and TET2 compared to those < 75 years. PTEN and TET2 are tumour suppressors and MYC is an oncogene with an important regulatory role in cell growth and proliferation. We hypothesize that hypo-methylating agents targeting TET2, BET inhibitors in MYC mutated tumours and PI3K inhibitors to target PTEN deficient lymphoma may help improve clinical outcomes in elderly DLBCL. No clear prognostic markers were identified in this small cohort.
Trotman:Celgene:Research Funding;BeiGene:Research Funding;Takeda:Research Funding;PCYC:Research Funding;F. Hoffmann-La Roche:Research Funding.Verner:Janssen Cilag Pty Ltd.:Research Funding.Gandhi:Celgene:Research Funding;Bristol-Myers Squibb:Research Funding;Mater Research:Current Employment;Janssen-Cilag:Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding;Roche:Other: Travel, accommodation, expenses ;Genentech:Honoraria;Gilead Sciences:Honoraria;Amgen:Honoraria;Merck Sharp & Dohme:Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.Gandhi:Integrated Sciences:Current Employment.Talaulikar:Takeda:Research Funding;Amgen:Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding;Janssen:Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau;Roche:Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal